Variants of Growth Hormone and Exercise

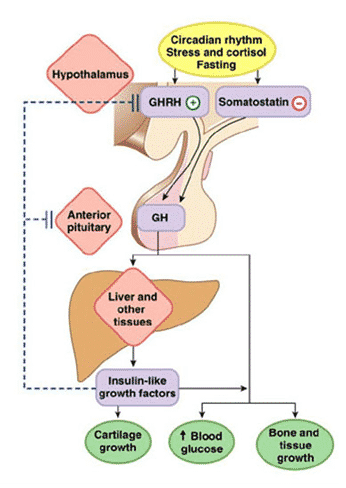
Exercise stimulates the secretion of somatotropin hormone (growth hormone ) of the anterior pituitary gland , and increasing the concentration of growth hormone in the blood contributes to the implementation of exercise-induced muscle hypertrophy and fat splitting, as well as other physiological reactions. An analysis of the literature on the relationship between the secretion of GH and physical training allows us to draw a general conclusion that the concentration of GH in the blood plasma is largely determined by the duration and intensity of the training sessions.
At the same time, it seems much less obvious that many are able to fully imagine the complexity of the processes that ensure the functioning of the growth hormone production system in the pituitary gland. Related issues are mainly discussed in articles published in journals for specialists, the main focus of which is on the secretion of growth hormone, which is not related to exercise. However, the situation is gradually beginning to change.
Below we consider the biochemical and physiological properties of the molecule STH and its various forms. Let us analyze the literature data demonstrating changes in the ratio of various variants of GH in the blood after exercise, also briefly touch upon some aspects of the cellular biology of the pituitary gland with the hope that this information will help form the fundamental basis for understanding how the products of various GHGs can be interconnected with the changes occurring in response to aerobic or strength training. And finally, we present data that clearly indicate the existence of a new chain of feedback between the muscles and the pituitary gland. The authors are confident that this newly discovered feedback mechanism will help explain the mechanism (s) underlying exercise-induced GH secretion.
Determining the growth hormone concentration
At present, plasma GH content is almost always measured by immunoassay. At the same time, there are other detection systems, but the choice of the method for determining GH in the blood still does not lose its importance. Before the advent of the GHG assay using immunoassay, researchers usually relied on biological detection methods for which rats were usually needed. Some of these techniques are used in laboratories where the authors of this chapter work today (Roth et al., 1963; Hunter, Greenwood, 1964). Since the use of these methods often allows to obtain interesting results that are not always consistent with the estimates obtained by the immunoassay method, we consider it justified to consider their details here.
Biological methods for determining CTT: experience of publications of previous years
These tests, which were developed over 50 years ago, reflect a growing knowledge of the anabolic, lipolysis, and diabetes effects of GH. The essential details of some of the tests used at that time are well described in the 1962 review (Papkoff, Li, 1962).
The following general requirements apply to tests for weight gain: a large number of rats are required (10 per dose level); injections can be made subcutaneously or intraperitoneally; normal rats can be used either with the pituitary gland removed; body mass assessment tests have a rather low sensitivity (a dose of 50 mg per day is required for adult females and 10 mg per day for immature rats with a pituitary gland removed); accuracy rate (calculated by dividing the standard deviation by the slope of the curve) exceeds 0.2; impurities of other hormones (for example, thyroxin) in the GH preparations derived from natural sources may have a synergistic effect, which will lead to an overestimation of the estimated values. Despite all these shortcomings, the traditional test for weight gain,
As for the tibia test or the test for determining changes in the width of the line of the epiphyseal cartilage of the tibia bone of rats under the influence of GH (tibia line GH bioassay, Greenspan et al., 1949), its main advantage over the test for increasing body weight is a markedly higher sensitivity (the reaction is detected when using a total dose of 5 mg for a period of 4 days). This test is used to determine the width of a non-calcified epiphyseal cartilage plate of the rat tibias bone, separated from the silver nitrate-colored calcified portion of the plate. This test was used by the authors of this chapter in conducting various studies, including those aimed at studying the effects of motor activity / bed rest on the level of GH in the blood.
The list of biological activities of human growth hormone is constantly expanding. As Strasburg (Strasburg, 1994) noted, it has long been known that GH is an anabolic protein that stimulates longitudinal bone growth. In addition, it was found that it has a lactogenic effect, agonistic and antagonistic properties in relation to insulin , has a lipolysis effect, stimulates ornithine decarboxylase in the liver, participates in the regulation of sodium and water metabolism, and also modulates the function of the immune system.
Tests using cultured cell lines of lymphocytes IM-9 and adipocytes ZTZ-F422A are relatively recent, noteworthy biological methods for determining GH in vitro on cells. According to our data, they were not used to assess the activity of growth hormone in plasma after exercise, so we do not consider them in more detail.
Biological methods for determining GH: new perspectives
In a paper published by Rossville (Roswell et al., 1996), a thorough comparison was made of two new biological methods for determining GH developed in the laboratories of the authors and a test for increasing the body weight of rats with pituitary removed. In order to fully appreciate the principles underlying these new methods, it is necessary to consider: a) the structural features of the growth hormone molecule obtained by genetic engineering (recombinant human growth hormone, HCG); b) its structural variants and degradation products; c) molecular interactions between these well-studied forms and the human GH receptor.
The primary structure of the recombinant human growth hormone and its molecular features
 The primary sequence of the form of recombinant human growth hormone, consisting of 191 amino acid residues (22 KG). This form is identical to the natural molecule STH mol. mass of 22 KG, which is synthesized in the pituitary gland and is released into the bloodstream with physiological needs. A distinctive structural feature is the position of cysteine residues. Responsible for the formation of a large internal disulfide loop and a smaller loop at the end of the c-protein. Also shown are enzymatic cleavage sites located between the residues of threonine-136 and tyrosine-143. Cleavage of the peptide molecule in this place leads to the formation of a structure consisting of two chains connected by disulfide bridges. The formation of such a double-stranded form can occur with the participation of membrane-associated protease during secretion of the hormone by the pituitary cells. With long-term storage of growth hormone in solution, deamidation of asparagine residues 149 and 152 can occur, as well as the loss of two extreme residues at the N-terminus.
The primary sequence of the form of recombinant human growth hormone, consisting of 191 amino acid residues (22 KG). This form is identical to the natural molecule STH mol. mass of 22 KG, which is synthesized in the pituitary gland and is released into the bloodstream with physiological needs. A distinctive structural feature is the position of cysteine residues. Responsible for the formation of a large internal disulfide loop and a smaller loop at the end of the c-protein. Also shown are enzymatic cleavage sites located between the residues of threonine-136 and tyrosine-143. Cleavage of the peptide molecule in this place leads to the formation of a structure consisting of two chains connected by disulfide bridges. The formation of such a double-stranded form can occur with the participation of membrane-associated protease during secretion of the hormone by the pituitary cells. With long-term storage of growth hormone in solution, deamidation of asparagine residues 149 and 152 can occur, as well as the loss of two extreme residues at the N-terminus.
In biological samples, various structural variants of growth hormone are detected. In their studies, Rossville and colleagues (Roswell et al., 1996) obtained two options from those found in nature, using recombinant growth hormone as a baseline. One of these options was a dimer, formed as a result of the formation of a covalent bond between methionine residues, the other is a transcriptional variant with a mole. Mass of 20 KG, resulting from deletion of residues 32-46. These options were used in the studies that are discussed below.
Knowledge of the features of the interaction of recombinant growth hormone molecules with membrane tissue receptors of growth hormone is of great importance for a deeper understanding of the significance and physiological consequences of increasing the level of growth hormone in the blood in response to exercise. Studies by Cunningham and his colleagues over 15 years ago not only established the complete amino acid sequence of the membrane somatotropin receptor, but also showed that the extracellular component is almost identical to the glycosylated form of the receptor isolated from human serum (Cunningham, Wells, 1989; Cunningham et al., 1991 ). These researchers have shown that one molecule of STG with mol. a mass of 22 KG forms a complex with extracellular receptors of two molecules of the GH receptor.
Knowledge of the molecular basis of this physiological interaction allowed Roseola (Roswell et al., 1996) to develop two different types of biological methods for determining GH. One called high-performance receptor binding chromatography(high performance receptor binding chromatography, HPRBC), is compared to the ability of the studied GHG sample and the standard hGST standard to form a stable 2: 1 receptor / GH complex with a soluble GH receptor. Size-exclusion chromatography was used to analyze the resulting complex under non-denaturing conditions. Strasburg and colleagues recently developed an enzyme-linked immuno-functional immunoassay (ELISA) based on earlier work by Cunningham and his colleagues (Strasburg et al., 1996). In this method, monoclonal antibodies to GH and biotinylated GH-binding protein are used to assess the functional activity of preparations containing growth hormone. ELISA was also used to determine the content of somatotropin in the circulatory system after exercise.
The second method proposed by Rossville (Roswell et al., 1996) is called the cell proliferation test.(cell proliferation assay, CP) and consists in the fact that the cells of the mouse myeloid leukemia cell line FDC-P1, transfected with the complete receptor gene, were incubated with the test samples containing GH and, as an indicator of the biological activity, proliferated cells by incorporating 3H-labeled thymidine in the DNA. A similar approach was used to determine the activity of various variants of STH using Ba / F3-hGHR cells (Wada et al., 1998). In these cellular tests, a response is evaluated (i.e., DNA synthesis as an indicator of cell proliferative activity), which is separated from the receptor dimerization process by several links of the signal chain. Rossville and his colleagues believe that thanks to this we can get “a few more steps closer to understanding the biological response in vim” (Roswell et al., 1996, p. 36).
Comparing the results of various methods
Comparison of the activities of genetic and chemical variants of recombinant somatotropin, determined using the rat body mass increase test, high performance receptor binding chromatography and cell proliferation test is a source of useful information that may be important when conducting a subsequent assessment of similar parameters in human blood plasma after exercise motor activity. The data table. (Roswell et al., 1996) demonstrate high activity and good agreement between the results of the evaluation of individual samples (for example, dimidiated STH and oxidized STH); low activity in the case of preparations of dimers or trypsin-treated STG, as well as “over-activity” in the test of weight gain in rats of the double-stranded variant of STG. As Rossville points out (Roswell et al.,
Although cell proliferation tests, described by Rossville and Vala (Roswell et al., 1996; Wadaet al., 1998), have yet to be used to study blood samples taken before and after exercise, it seems very likely that In the near future, they will play an important role in the study of the functional role of somatotropin hormone.
A variety of somatotropin hormone forms circulating in the circulatory system
 Bauman suggested that up to 100 different forms of somatotropin can be detected in human blood (Baumann, 1991b). The concept that the numerous molecular forms of growth hormone can arise as a result of post-translational or translational modifications of the expression product in the pituitary gland of a single GH-N gene is obviously not new. Pioneering work carried out in the laboratories of Lewis, Siah, Kostou, Bauman and other researchers provided the basis for the subsequent analysis carried out in the article by Baumann (Baumann, 1991b). In the table., Borrowed from this work, presents figures that characterize the percentage representation of various forms of growth hormone 15 minutes after secretion. Many studies, the results of which summarizes this table, were carried out before recombinant technologies became widespread. No wonder therefore
Bauman suggested that up to 100 different forms of somatotropin can be detected in human blood (Baumann, 1991b). The concept that the numerous molecular forms of growth hormone can arise as a result of post-translational or translational modifications of the expression product in the pituitary gland of a single GH-N gene is obviously not new. Pioneering work carried out in the laboratories of Lewis, Siah, Kostou, Bauman and other researchers provided the basis for the subsequent analysis carried out in the article by Baumann (Baumann, 1991b). In the table., Borrowed from this work, presents figures that characterize the percentage representation of various forms of growth hormone 15 minutes after secretion. Many studies, the results of which summarizes this table, were carried out before recombinant technologies became widespread. No wonder therefore
The literature presents a lot of experimental data that allow us to characterize the chemical nature of various variants of growth hormone. A short but far from complete analysis of these works is very important for the internal understanding of the mechanisms that ensure the heterogeneity of GH and the formation of its complexes, which can take part in the body’s response to physical activity and adaptation. The fact that immunoreactivity somatotropin plasma hormone includes several types of molecules with different mol. weight, which can be divided exclusion chromatography, it was known more than 30 years ago. Depending on the order of their elution from the column in the past, it was convenient to distinguish three main isomers (variants) of GH: small, large, and very large. The physical nature of these variants of growth hormone is much worse compared to research, in which the recombinant protein was used. Despite this, detailed studies by two groups of scientists under the leadership of Bauman and Lewis (Baumann, 1991a, 1991b, 1999; Baumann et al., 1994; Lewis et al., 2000), aimed at characterizing large and very large forms of the hormone, allowed to conclude that these options are a series of oligomers. The presence of the same oligomers in extracts of the human pituitary also confirms this point of view; therefore, most scientists believe that the formation of at least pen tamer complexes can occur during aggregation and the differences between large and very large GH variants are rather arbitrary. Researchers prefer to break oligomers into groups depending on their molecular weight, determined on the basis of the elution profile during chromatography on Sephardic. In addition to the oligomers STG,
Studies by Solar (Solar et al., 1984) also show that the bulk of a large and very large variant of GH turns into a small form of GH with mol. 22 KG in extraction and storage (for example, exposure to 4 M potassium thiocyanate [KSCN] and two freeze-thaw cycles lead to the conversion of 70% of oligomers into somatotropin monomer) Oligomeric forms of the hormone, which survived after such a harsh treatment, migrate as separate bands with a mol. weighing 45, 62, 80 and 110 KG. These forms are quantitatively (almost completely) transformed into a small form of the hormone after the reduction of sulfhydryl groups. A small part of the product of this reaction is the acidic form of GH. Option STG mol. weighing 20 KG forms mainly dimers.
What is known about the biological activity of enzyme oligomers? In general, according to the results of tests with radio receptors and in rodents, it is believed that a large form (dimer) has a reduced activity. At the same time, according to the enzyme immunoassay data (Strasburg et al., 1996), dimers with an equal molar concentration have a higher activity (110%) compared with the monomer, mol. the mass of which is 22 KG.
The properties of five different variants of somatotropin are described in a recent review of the results of the work of the Lewis and Sinha groups (Lewis et al., 2000). Two of them are short and long peptides, formed as a result of proteolytic cleavage of the growth hormone molecule between the 43rd and 44th amino acid residues. Data from these researchers argue in favor of the concept that a short peptide (STG [1–43 |) enhances the physiological effects of insulin, and a long peptide (STG | 44–1911) has anti-insulin properties. In fact, they write: “We believe that this (larger) peptide is the diabetes hygienic product of the pituitary gland, which for a long time could not be found.”
In the extracts of the pituitary glands of the dead and in the blood plasma, a peptide was found with a mol. weighing about 3 KG, which is active in tibia test on rats (Hymen et al., 2000). This peptide is not a fragment of the growth hormone. The association of this protein with the various forms of growth hormone described by Bauman remains unclear (Baumann, 1999). The incomplete sequence of this protein, which contains the 9-25th amino acid residues from its middle part, shows that it cannot be a product of the splitting of growth hormone. Most interestingly, many of these amino acid residues in its composition are non-polar, and in general the sequence is very similar to one of the sections of the proinsulin molecule. Like peptide C, this peptide secreted by the pituitary gland undoubtedly also has biological activity. According to unpublished data of one of our laboratories (R.G.),
Various forms of growth hormone: an increasing amount of data
Despite the fact that for many years it has been known about the stimulating effect of physical exercise on the level of GH in the blood, it was only recently that a question was raised about the possible change in the ratio of various forms in the circulatory system under the influence of physical activity (Nindl et al., 2003). Consider some preliminary data obtained by us in the context of the information discussed above. To analyze in a logical way and obtain results, the following variables should be considered: the direction of research; type of exercise (intensity / duration); type of determination of GH; the method used to isolate individual forms of the hormone; special processing of blood samples.
In tab. summarized the results of studies divided into separate groups (in which only people participated) in accordance with the conditions we chose – in each case, the sample was studied, at least by two methods, in order to deepen understanding of exercise-induced increase in GH level in the blood, as well as assess the quantitative ratio of various hormone. Only in one study (Hymer ct al., 2001), fractionated plasma was used to quantify the GH variants, but all other studies studied only the complete plasma.
Experimental data accumulated to date indicate that exercise can alter the activity or molecular composition of GH in the blood. Wallace and his colleagues used seven different methods for quantifying GH in 17 men doing aerobic training, before and after 20 minutes of bicycle ergometer at 80% V02max, to assess changes in the content of various molecular isoforms under the influence of physical activity (Wallace et al., 2001) . The blood serum was analyzed with specific antibodies to the total, pituitary, form 22 KG, recombinant, not containing the form 22 KG, form 20 KG and immune functional (IF) GH. The main results of this study were the conclusions that: a) during and after exposure to physical exercise, there is an increase in the blood content of all forms of growth hormone; b) after the cessation of exercise, the predominant isoform was 22 KG STG with a mole. weighing 73%; c) the ratio “STH without form with 22 KG” / “total GHG” and “20 KG STH” / “total GHG” increased, and the ratio “recombinant STH” / “pituitary GHG” decreased. Wallace (Wallace et al. , 2001) explained the increase in isoforms other than 22 KG growth hormone, the slower disappearance of 20 KG and possibly other (except 22 KG) forms of the hormone. In general, the results obtained by Wallace show that under the influence of intense physical exercise and during the recovery period there is a change in the ratio of different isoforms of STH. Despite the fact that T with mol. Weight of 22 KG was the predominant molecular isoform detected in highest concentrations, during the recovery period after exposure to exercise, other growth hormone isoforms increased. This suggests that the relative content of the forms with a mol. a mass of 20 to Yes, 17 KG, as well as forms with a mol. weighing more than 22 KG (dimers, oligomers and complexes with sulfur-containing proteins) after exercise increases. The authors suggested that an increase in the proportion of isoforms with a mol. a mass different from 22 KG in the period after exposure to physical activity may be due to the differential secretion of various isoforms of the hormone by the pituitary, the formation of fragments, dimers and oligomers in the circulatory system, as well as different rates of clearance of different forms of the hormone. The authors also suggested hypoglycemia in the period after exercise.
Continuing the experiments started by Wallace (Wallace et al., 2001), Chimer, Kremer, and Nindl conducted a study in which plasma taken from 35 women before and after intense physical activity (6 sets of squatting’s with a 10 PM squat, for intervals between 2 min.), were fractionated by exclusion chromatography on a fi size class (Hymen et al., 2001). Fraction A contained molecules with mol. more than 60 KG (presumably oligomers and / or monomeric GH associated with the receptor); fraction B contained molecules with mol. weighing 30–60 KG (presumably homo-and heterodimers), and the composition of fraction C consisted of GH molecules with a mol. weighing less than 30 KG (presumably a mixture of isoforms with a mol. mass of 22, 20, 16, 12 and 5 KG). After that, all samples were analyzed using Diagnostic Systems Laboratory IFA, radioimmune physical analysis (Nichols IRMA) and radioimmunoassay (National Institute for Diabetes and RIA). In addition, all samples were analyzed before and after glutathione (GSH) treatment in order to determine the effect of chemical reduction of disulfide bonds. The definition of immunoreactivity showed that for fraction A this figure was 4–11% of the total plasma GH, for fraction B – 22–45% and for fraction C – 44–72%. A significant increase in this index, induced by exercise, was found for low molecular weight forms of growth hormone (30–60 KG and less than 30 KG), but not for the high molecular weight fraction of the hormone (more than 60 KG). Another important result was the fact that the chemical reduction of samples taken after exercise led to an increase in immunoreactivity STH, according to Nichols IRMA and KIDDKD RIA tests, more significant than was observed for samples taken before class. This suggests that exercise can specifically increase the secretion of hormone molecules and / or their fragments linked by disulfide bridges. According to this study, the most significant effect of intense physical exertion is on the dimeric form of the hormone. Since the complexes of GH and GH-binding protein have a longer lifespan compared with the free hormone, it is likely that the dimeric form also has a longer lifespan. In this way,
The work of Nindl and his co-authors (Nindl et al., 2000) presented the results of a comparison of the effects of physical activity on immune functional (IF) GH compared to immunoreactivity (MI) GH. The concentrations of IF and IR STG were compared in men and women before and after training with intensive strength exercises (i.e., 6 squatting approaches with a load of 10 PM and a duration of 2 minutes between them). The concentration of IF STG was determined using enzyme-linked immunosorbent assay (ELISA, Diagnostic Systems Laboratories, Webster, TX, USA), which was developed based on the results of Strasburg (Strasburg et al., 1996), and the concentration of IL STH using RIA with monoclonal antibodies ( Nichols IRMA, San Juan Capistrano, CA, USA). In this work, both women and men demonstrated a similar increase for IR (men: 1, 47 compared to 25.0 ng-ml_ |; women: 4.0 compared to 25.4 ng-ml-1) and for IF (men: 0.55 compared to 11.7 ng-ml-1; women: 1.94 compared to 10.4 ng-ml-1 A) growth hormones after exercise. At the same time, the content of IF STG was significantly lower than that of IR STG, in both men and women. The correlation between the values of IF STG and IR STG after exercise was r = 0.83. One of the conclusions of this study was that about half of the isoforms of GH, detected by radioimmunoassay (Nichols IRMA), are characterized by the absence of free binding sites 1 and 2, which are necessary for dimerization of the receptor, which may indicate the absence of biological activity of the biological isoforms of the hormone. 55 compared to 11.7 ng-ml-1; women: 1.94 compared with 10.4 ng-ml-1) STH after exercise. At the same time, the content of IF STG was significantly lower than that of IR STG, in both men and women. The correlation between the values of IF STG and IR STG after exercise was r = 0.83. One of the findings of this study was that about half of the isoforms of GH, detected by radioimmunoassay (Nichols IRMA), are characterized by the absence of free binding sites 1 and 2, which are necessary for dimerization of the receptor, which may indicate the absence of biological activity of the biological isoforms. 55 compared to 11.7 ng-ml-1; women: 1.94 compared with 10.4 ng-ml-1) STH after exercise. At the same time, the content of IF STG was significantly lower than that of IR STG, in both men and women. The correlation between the values of IF STG and IR STG after exercise was r = 0.83. One of the findings of this study was that about half of the isoforms of GH, detected by radioimmunoassay (Nichols IRMA), are characterized by the absence of free binding sites 1 and 2, which are necessary for dimerization of the receptor, which may indicate the absence of biological activity of the biological isoforms. The correlation between the values of IF STG and IR STG after exercise was r = 0.83. One of the findings of this study was that about half of the isoforms of GH, detected by radioimmunoassay (Nichols IRMA), are characterized by the absence of free binding sites 1 and 2, which are necessary for dimerization of the receptor, which may indicate the absence of biological activity of the biological isoforms. The correlation between the values of IF STG and IR STG after exercise was r = 0.83. One of the findings of this study was that about half of the isoforms of GH, detected by radioimmunoassay (Nichols IRMA), are characterized by the absence of free binding sites 1 and 2, which are necessary for dimerization of the receptor, which may indicate the absence of biological activity of the biological isoforms.
In the next experiment it was taken into account that the secretion of growth hormone is not constant, but has a pulsating character. The content of IF of STG was determined in 10 men, taking blood for analysis every 10 minutes from 17.00 to 6.00. The experiment was repeated twice. Blood sampling was carried out in the control group and in individuals exposed to intensive physical exercise (Nindl et al., 2001). Physical IA-Fuzka consisted in the implementation of strength exercises with a significant amount of trainers in the period from 15.00 to 17.00. IF GH was determined by radioimmunoassay and ELISA with polyclonal antibodies. To characterize the pulsatile nature of the GH secretion, the Pulsar peak detection system was used. Despite the significant correlation of the assessment results using all three methods (the correlation coefficient ranged from 0.85 to 0.95), The radioimmunoassay method again showed a higher average concentration of growth hormone compared with IFG STG (3.98 and 1.83 ng-ml ”1, respectively). The values of the maximum amplitude of the peaks of GH secretion, determined by the RIA method, also turned out to be higher compared with the ELISA estimates (8.0 and 4.63 ng-ml-1, respectively).
A common feature of all these studies (Nindl et al., 2001, 2002, 2003) was that for the same sample, the GHP IP estimate was about half of the GH value determined by the RIA method (Nichols IRMA), which is one of the most common methods for the quantitative determination of growth hormone when conducting medical tests in the United States. Since ELISA allows determining only biologically active forms of somatotropin hormone (i.e., only forms of GH that are able to induce receptor dimerization and subsequent signal transmission), additional GH isoforms that are detected by RIA are most likely fragments whose biological activity is realized the participation of GH receptors. It has been reported that fragment 44 – 191 is found in human serum in significant quantities and may even have an antagonistic effect on GH (Rawlinson et al., 1996). Since this fragment is devoid of the N-terminal part of the peptide chain, it is most likely not detected by the IF analysis. At the same time, it will be detected by RIA depending on the epitopes recognized by the antibodies. It is also possible that the additional STH isoforms detected by RIA are high-molecular-weight GH complexes (Baumann et al., 1991a; Lewis et al., 2000).
Our studies have finally proved that at least part of the molecules released into the bloodstream at the moments of maximum GH secretion can initiate the dimerization of GH receptors, in this sense, it has biological activity. On the other hand, our data show that GH isoforms are secreted both in the control group and after exposure to intense physical activity, which are not able to transmit a signal through growth hormone receptors. A significant correlation of the results of immunoassay and other methods for detecting the number of secretion peaks and the intervals between them suggests that ELISA makes it possible to obtain a qualitatively comparable pattern of fluctuations in the level of GH. The reasons for the quantitative differences in the results of the STH assessment by different methods have yet to be clarified, but it can be assumed that they are due to the existence of various molecular isoforms of the hormone. In addition, features of the reaction conditions, the buffers used, the indicator compounds and the standard samples (Wood, 2001) can contribute to the differences in the results of the quantitative assessment of GH.
When conducting ELISA, the effect due to the presence of GH-binding proteins should be taken into account.(Strasburg et al., 1996; Nindl et al., 2001). In the ELISA, for binding to site 1, the growth hormone molecule uses recombinant growth hormone receptor (recombinant growth hormone binding protein, GHBP). It can be assumed that this analysis system will not be able to detect GH molecules that are already in the complex with GHBP, since the binding site 1 will be inaccessible. In addition, during the formation of a complex of GH with a binding protein, access for binding to site 2 of monoclonal antibodies (mAb7B11) can be closed. It was reported that high-affinity GHBP can suppress GH binding to receptors and the manifestation of biological activity in in vitro experiments by competing for ligand Strasburg et al., 1996). If the statement is true
Comparison of the results of RIA (IRMA, Nichols) and ELISA (IFA, DSL) was conducted in another study (Rubin et al., 2003), in which 6 men were involved in training aerobic endurance. During the treadmill session, the load was gradually increased as follows; 60% VO2max – 10 min; 75% – 10 min; 90% – 10 min; 100% – 2 minutes The samples were analyzed before and after treatment with glutathione (GSH, 10 mM for 18 h at room temperature), designed to break the disulfide bonds between possible GH oligomeric complexes. According to RIA, the concentration of somatotropin increased after increasing the intensity of the load to 75% V02max and remained elevated for 30 minutes after the completion of the session. When analyzing samples treated with GSH, RIA detected an increase in the concentration of growth hormone at a load intensity of 60% V02max, and maintaining a high level of the hormone – for 45 minutes after the cessation of the session. At a loading intensity of 75%, the GHR quantitative estimates by the RIA method were higher for samples treated with GSH. When conducting enzyme immunoassay, samples not treated with glutathione, an increase in the level of growth hormone was observed already at a loading intensity of 60%, whereas in samples incubated with GSH, an increase in the concentration of growth hormone was detected only when the intensity of the filling was increased to 75% V02max. In both groups of samples (treated and untreated GSH), elevated levels of somatotropin were observed for 30 minutes after the exercise was completed. These results suggest
Tibia test results and response to exercise
Addressing the discrepancies in the results of assessing the concentration of growth hormone using tibia test (determining the width of the epiphyseal cartilage of tibias rats after administration of growth hormone) and immunoassay is important when planning future experiments aimed at clarifying the relationship between different STH isoforms and exercise. It is very important to consider the total GH as a set of isoforms that react with high affinity antibodies to the “native” form of somatotropin hormone described in textbooks with a mol. 22 KG in weight (measurable immunoassay for somatotropin, iSTG), and those isoforms that do not react with these antibodies, but stimulate growth processes that can be detected in biological tests (biologically assessable somatotropin, 6CTG). Quite possibly,
At present, it seems that Tidiest is preferable in the case of an assessment of the “functional status” of the STH in the sample under study. Despite the laboriousness, as well as significant financial and time costs, this test will provide information that cannot be obtained in any other way. There is no doubt that the modern researcher or doctor is more comfortable and understandable to work with the data of the plasma GH assessment using immunoassay. However, the calculated concentrations of 6CTG, mentioned in scientific publications, often amount to hundreds or even thousands of nanophases per milliliter! Why is this so? This is because this method of biological determination of GHG evaluates biological activity, and not monograms of purified hormone. Here it is important to understand that purified STG, obtained from various sources (human, bull; mouse), and its 6STG give parallel dose-response curves in the evaluation of tests. Similar curves, describing the dependence of the growth of the epiphyseal plate on the amount of GH applied, make it possible to express the biological activity of the hormone in the form of the corresponding amount of the standard preparation of somatotropin, mol. weighing 22 KG.
Anyway, it is very important to give at least some assessment of the complexity and significance of determining the most important assessment of GH in this physiological context. Moreover, the following two examples of comparing 6STG and ISTG assessments can dispel any possible skepticism regarding the definition of 6CTG by Tibia testiest.
Differences between evaluated biological tests, somatotropin hormone and CTT immune analysis
Rats have played an important role in the development of our modern concepts and the differences between STG and IAST. For example, many researchers report that stimuli (for example, low temperatures, hypoglycemia, physical exertion), which cause an increase in the concentration of GH in the blood plasma of a person, do not have any effect on the level of IGH in Crops. Studies by one of the authors of this article (RG) made it possible to establish that in rats in response to these stimuli, there is secretion of forms of GH which are not recognized by antibodies to GH of a rat with a mole. weighing 22 KG (Ellis, Grind eland, 1974). However, despite the lack of immunoreactivity, these forms of the hormone cause a significant increase in experimental rats (Ellis, Grind eland, 1974).
The results obtained make it possible to make a general, although still speculative, conclusion that despite the absence of a clear relationship between ISTG and 6CTG in rats, fluctuations in the ratio of biologically active / immunoreactivity human GH in biological samples tend to change in the same direction. However, since the titer of ISTG and 6CTG of a person is not directly proportional to each other, we believe that the results of the evaluation of ISTG cannot be used as an indicator of the total content of GHG in the blood.
Almost coincided with these early studies of rat and human 6CTG, detection of plasmin that showed protease activity in relation to GH of higher animals (rat, bull), which led to a decrease or elimination of the immunological activity of the hormone from mol. mass of 22 KG (Ellis et al., 1968). However, as a result of this treatment, peptides were formed with normal or even increased biological activity. Other researchers have shown that human ISTG after treatment with human plasmin does not lose immunoreactivity, but increases biological activity (Singh et al., 1974; Lewis et al., 1975; Nguyen et al., 1981). It is clear that the ratio immunoreactivity / biological activity of the molecule STH mol. mass of 22 KG after enzymatic treatment may vary significantly.
Studies on the effect of bed rest on blood levels of GH
For those who perform physical exercises with a small load (for example, bending the feet) for a few minutes, there is a one-to-two-fold increase in 6CTG in the blood, while the content of ISTG remains almost unchanged. However, in the case of absolute bed rest, when the body is in a horizontal position, the same physical activity is not accompanied by any changes in 6STG and ISTG (McCall et al., 1977). Interestingly, a few days after the cessation of bed rest, the ability to increase 6STG secretion in response to physical exercise is restored.
What can these results mean for different types of growth hormone and exercise? In studies on the physiology of motor activity, changes in the concentration of metabolic regulators in the blood are considered as the main mechanism for stimulating an increase in GH secretion in response to an increase in muscle activity. Interestingly, none of the most frequently mentioned metabolic factors (such as lactate, blood glucose) can explain the decrease in 6STG plasma concentration. Such a clear discrepancy has raised in one of the authors of this chapter the question of the existence of a mechanism for the nervous regulation of GH secretion. The answer to this question is most likely “yes,” but here physiologists must say the final word.
Afferent muscular nerves regulating the release of somatotropin hormone in rats
In the initial studies, animals were used in which nerves that innervate the hind limbs were cut. During electrical stimulation of the distal end of the nerve for 15 minutes with pulses resembling those that occur in a rat running at a speed of 2.4 km per hour, no change in the content of 6STG and GH in the blood plasma and pituitary was found (Gosse link et al ., 1998, 2000; McCall et al., 2000).
At the same time, after stimulation of the proximal end of the transected nerve, which innervates the rapidly contracting muscle fibers, a significant (one-, two-fold) increase in plasma 6STG was observed after 5 min! It is also significant that the increase in plasma 6CTG level was accompanied by a significant decrease in the concentration of 6STG in the pituitary gland. At the same time, no changes in the content of hSTG were detected either in the blood plasma or in the pituitary gland. No less interesting is the fact that stimulation of the proximal nerves of the soleus muscle led to a decrease in plasma 6CTG concentration. This observation suggests the presence of specificity of action of muscle groups in this afferent path.
The results obtained are of interest from two points of view. First, they confirm the existence of the mechanism of the nervous regulation of the pituitary system of the synthesis of GH, which functions along with the metabolic system of regulation. Secondly, we believe that these experiments allow us to re-evaluate the physiological significance of 6CTG. If we assume that one of the most important functions of GH is to provide a constant supply of glucose to the heart and brain, then these results fit into the framework of the existing concept. A significant release of GHH in response to an increase in metabolic needs (for example, fasting, hypoglycemia, or a lower ambient temperature) and an increase in GH secretion in response to activation of the resting muscle responsible for movement suggest mechanisms for ensuring increased glucose uptake by tissues.
It is known that human secretion of ISTG increases in response to physical exertion, but this response is found only 15–20 minutes after the start of motor activity. Suppose that at rest, the gastrocnemius and other postural muscles (responsible for maintaining posture), whose activity remains 80% even in mowing, with the help of the afferent innervation system, give the pituitary a signal to decrease 6CTG production, thanks to which other tissues of the body, but not only the brain and the heart are able to use glucose as a source of energy. When activating locomotor (setting the body in motion) muscles, the pituitary receives a signal that stimulates 6CTG production. The net effect is supposedly to limit the use of glucose by active people and to stimulate their transition to other energy sources such as
In fig. We propose a model adapted from our publication (McCall et al., 2001), which describes a muscle-neural feedback circuit that regulates GH secretion by the pituitary gland. In this figure, nerve impulses enter the hypothalamic neurons. However, it is likely that they can flow directly into the anterior pituitary gland. There are very few scientific publications mentioning innervation of the pituitary gland. A relatively recent paper (Paden et al., 1994, p. 503) refers to “surprisingly extensive innervation of the anterior pituitary gland”. Interestingly, the nerve endings that innervate the pituitary gland are often associated with blood vessels and do not resemble ordinary vasomotor nerve fibers. Their distribution is rather uneven, it seems ACTH ).
Acute and chronic effects when performing strength exercises and biologically active somatotropin hormone
In a recent study, we (Kremer, Chimer, and Nipple) studied the effect of intense strength training (i.e., 6 squatting approaches with a load of 10 PM with an interval of 2 minutes for rest) on the 6CTG level before and after 6 months of per iodized strength training in young healthy women. The results of this study show that although intense physical activity does not lead to changes in the level of 6STG in the blood, after 6 months of regular strength training, there is a noticeable increase in the level of 6CTG. These results suggest that one of the positive effects of prolonged regular strength training sessions is an increase in the biological activity of somatotropin hormone in the circulatory system. The ego is a new discovery, perhaps one of the mechanisms
Conclusion
The main points that we have tried to illustrate in this chapter can be summarized as follows.
- Molecules STH heterogeneous. This chapter defines and discusses various aspects of GH heterogeneity. These include: a) variants of hormone molecules with different molecular weight and size, which are the product of the expression of a single pituitary GH gene; b) heterogeneity due to different hormone activity, which can be determined by the response they cause: biological Vg vivo) or immunological (in vitro) ‘, c) heterogeneity of pituitary cells that produce and secrete GH molecules.
- Aerobic and strength exercises can lead to the differential secretion of various isoforms of somatotropin hormone into the bloodstream. Today there are only separate studies devoted to the analysis of changes in the molecular composition of growth hormone molecules occurring in response to physical exertion. At the same time, they all support the view that physical nausea stimulates the secretion of oligomeric forms of growth hormone with a molecular mass of 22 KG. The results of the assessment of the level of growth hormone by biological methods and immunoassay often do not coincide. The intensity and duration of the exercises play a major role in this split activity. After physical training at rest, an increase in the level of growth hormone occurs, which stimulates bone growth in rats.
- The cell system of production of growth hormone in the pituitary gland of a rat is heterogeneous. For the human pituitary gland, such heterogeneity of cells producing GH can also be characteristic, but it is rather difficult to conduct studies that would allow proving this position. Experimental data show that secretory granules containing GH, as well as cells that produce GH, differ from each other. Such heterogeneity, obviously, has a certain biological meaning. To establish the relationship between these components in rats and humans when exposed to physical exertion, more research is needed.
- The regulatory mechanisms responsible for the secretion of growth hormone variants by the pituitary gland may include signals from nerve endings located in the muscles that are stressed during exercise. In this chapter, we present evidence of the existence of a new feedback loop between certain muscles and the pituitary gland. It is likely that this chain exists in humans and in rats. This regulatory system may be an important factor in controlling the production and secretion of various hormone isoforms in the pituitary gland.
In conclusion, I would like to note that the readers of this chapter probably know that the information explosion, which we are now experiencing in the biological sciences, is the result not only of research of past years, of the rapid development of technology, but also an increase in the amount of accumulated data. It is obvious. The authors analyzed the fruitful studies conducted almost 50 years ago and tried to show that they have not lost their significance even today, helping more fully, to evaluate the role that various molecular forms of growth hormone can play in the positive effects of exercise on the human body. We tried to show that the successful combination of experimental approaches that are used in endocrinology, biochemistry, cell biology and physiology of motor activity, allows you to take a fresh look at the value that molecular heterogeneity of growth hormone in exercise can have. There is no doubt that a start has been made here, but much more needs to be done.